. Introduction
Dating of hydrothermal deposits is an important issue in the studies of the evolution of the sea floor hydrothermal fields where geochronological dating methods applied to hydrothermal deposits are the key techniques. Dating methods using radioactive disequilibrium have been employed for hydrothermal sulfide deposits, such as the U-Th method (e.g. You and Bickle,1998) for sulfide minerals applicable for the age range more than several thousand years, and 226Ra-210Pb and 228Ra-228Th methods (e.g. Noguchi et al., 2011) for barite extracted from sulfide deposits for the range less than 150 years. Although the age range of several hundred years is essential to estimate the life time of hydrothermal activities, dating methods for this age range were lacking.
Kasuya et al. (1991) investigated the dose responses of the electron spin resonance (ESR) signals observed in natural barite samples and first pointed out that ESR dating of barite is possible. Following Okumura et al. (2010), who made the first practical application of ESR dating to a sample of seafloor hydrothermal barite with the signal due to
Takamasa et al. (2013) examined sulfide deposits in the South Mariana Trough sea-floor hydrothermal field to compare the ESR ages obtained for barite and the U-Th ages for sulfide minerals extracted from the same portions. They showed that the ESR ages are roughly consistent with the U-Th ages. While Toyoda et al. (2014) discussed several detailed technical issues such as the dose rate conversion factors, the sample edge effect for gamma ray dose, and the correction for the decay of 226Ra, Fujiwara et al. (2015) made the first practical systematic dating work with ESR on barite of sea-floor hydrothermal sulfide deposits taken at Okinawa Trough on total of 18 samples (60 subsamples) with new formulas which take the radioactive disequilibrium of Ra daughter nuclei into account. The obtained ages range 4 to 16000 years, showing that the ESR method has a wide application age range. Uchida et al. (2015) obtained 226Ra-210Pb and 228Ra-228Th ages for the same samples as Fujiwara et al. (2015) analyzed and attributed the differences of the ages among these three dating methods to possible multiple formation stages of the sulfide deposits.
. Issues in determining dose rates to barite extracted from sea-floor hydrothermal sulfide deposits
When hydrothermal fluid containing barium ions is mixed with sea water, they react with sulfate ions in the sea water to precipitate barite (barium sulfate, BaSO4). The barite crystals occur in sulfide chimneys and in deposits, incorporating radium ions that are in the hydrothermal fluid. As a radium ion has an ionic radius similar with barium, radium ions easily replace barium in barite to have high concentration up to several tens Bq/g of 226Ra (Fujiwara et al., 2015).
The source of natural radiation in sulfide deposits containing barite is radium and its daughter nuclei in barite where the doses from the other minerals are negligible (Toyoda et al., 2014). Under these circumstances, the specific issues for the dose rates in calculating the ESR ages are, as summarized by Toyoda et al. (2014), the conversion factors, the calculation of internal and external dose rates including the determination of the alpha effectiveness, the water content, the geometric factors such as the grain sizes, and the radioactive disequilibrium. The radioactive disequilibrium is one of the most complex and important issue in calculating the ESR ages.
While Toyoda et al. (2014) devised a formula to correct the age due to the decay of 226Ra assuming radioactive equilibrium, Fujiwara et al. (2015) proposed formulas to consider the radioactive disequilibria. The same equation applies both to 226Ra and 228Ra series, and the total accumulated natural equivalent dose, DE(T), which is the summation of the two, is expressed by,
(2.1)
where λ1, λ2, λ3, and λ4 are the decay constants of 226Ra, 210Pb, 228Ra, and 228Th, respectively, Q1, Q2, Q3, and Q4 are the conversion factors of 226Ra to 214Po, 210Pb to 206Pb, 228Ra and 228Ac, and 228Th to 208Pb, respectively, referring to Guérin et al. (2011). λ1Nlp and λ3N3p are the present activity of 226Ra and 228Ra, respectively, and T is the age. When the experimentally obtained equivalent dose is Dexp, the equation is,
In order to solve this equation, a function, f(T) is defined as,
and the equation to be solved is deduced to
This non-linear equation can be numerically solved by the Newton method, which is
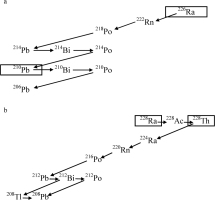
It should be noted that 210Pb has the longest half-life of 22.3 y in the decay series after 226Ra with a half-life of 1600 y (Fig. 1a), and that 228Th has the longest half-life of 1.91 y in the decay series after 228Ra with a half-life of 5.75 y (Fig. 1b), therefore, other radioactive disequilibrium can be neglected.
For the samples, in which 228Ra is observed, the contributions to the natural accumulated dose from the 228Ra series as well as that from 226Ra series can be calculated by Eq. 2.1 where the age, T, is obtained as the solution of, Eq. 2.4, as these procedures are actually adopted in obtaining the ESR ages of hydrothermal barite in Okinawa Trough (Fujiwara et al., 2015). As time passes, 228Ra with a half-life of 5.75 y will decay out so that 228Ra and its daughter nuclei will not contribute doses any more. Therefore, if the sample is old enough, the contribution of 228Ra and its daughter nuclei to the natural accumulated dose of barite would be negligible. However, the fact that 228Ra and its daughter nuclei are not observed would not necessarily guarantee that their contribution is negligible. In the present paper, the evolution of the contribution of each nucleus to the accumulated barite dose is simulated by numerical calculation to find that there is an age range in which the contribution from non-observable 228Ra and its daughter nuclei cannot be neglected.
. The analysis for the test sample
The simulation of the contribution from 228Ra and its daughter nuclei to the accumulated dose is made for an actual test sample, HPD1621R03, taken at a sea-floor hydrothermal area at depth of 1495 m of Hatoma Knoll, Okinawa Trough by the research cruise, KY14-02, operated by Japan Agency for Marine-Earth Science and Technology (JAMSTEC). The sample is a portion of sulfide deposit including barite crystals.
The analysis was made according to the procedures described in Fujiwara et al. (2015) and in Uchida et al. (2015). A piece of a block cut out of the deposit was soaked in water for 3 days and its mass was measured. After drying in an oven at 60°C for several days until the weight did not change, the water mass was estimated from the difference in the masses as the ratio of the mass of water to the dry mass to be 30.3%. The sample was then crushed and the bulk concentrations of 226Ra and 228Ra were obtained by low background gamma ray spectrometry with a pure Ge detector from the gamma ray peaks due to 214Bi and 214Pb, and 228Ac to be 16.9 Bq/g and 1.02 Bq/g, respectively.
The barite crystals were extracted from the crushed bulk sample by using HNO3 and HF. An XRF measurement was made to confirm that the extracted crystals are pure barite. The mass of barite crystals is 70.0% of the total dry mass before the chemical treatment. The barite grains were separated into 10 aliquots for gamma ray irradiation for the additive dose method. An ESR signal due to
Table 1
Experimental results for HPD1621R03 to be used for the simulation of the contribution of 228Ra and daughter nuclei to the accumulated doses in barite.
. Simulation for the contribution of the dose from 228Ra and daughter nuclei
The contribution of the radiation doses from 228Ra and its daughter nuclei to the accumulated dose to barite in this test sample, HPD1621R03, is simulated as a function of time as in the following. Based on the ESR age obtained as above, the initial (when the age of the sample is zero) bulk concentrations of 226Ra and 228Ra are calculated to be 17.0 Bq/g and 59.4 Bq/g, respectively.
The accumulation of the doses to barite given by the nuclei, 226Ra to 214Po, 210Pb to 206Pb, 228Ra and 228Ac, and 228Th to 208Pb are respectively calculated from the following formulas, which are deduced from Eq. 2.1,
(4.1)
where DE1, DE2, DE3, and DE4 are the doses from 226Ra to 214Po, 210Pb to 206Pb, 228Ra and 228Ac, and 228Th to 208Pb, respectively, and N10 and N30 are the initial concentrations of 226Ra and 228Ra, respectively.
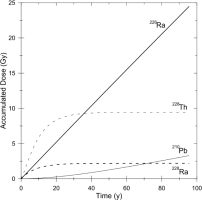
The values of DE1, DE2, DE3, and DE4 are calculated as a function of time for barite crystals in the sample as shown in Fig. 2. The accumulated dose from 226Ra to 214Po (indicated by 226Ra in the figure) linearly increases with time as the half-life of 226Ra, 1600 y, is much larger than the time scale shown in the figure. The one from 210Pb to 206Pb (indicated by 210Pb) slowly increase and then turns linear increase after equilibrated with 226Ra. The one from 228Ra and 228Ac (indicated by 228Ra) saturates rapidly meaning that those nuclei decay out rapidly where the half-life of 228Ra is 5.75 y. It is noted that the dose from 228Th to 208Pb (indicated by 228Th) gives a significant contribution when the sample is young. When their parent, 228Ra, decays out, these nuclei also decay out to give no contribution anymore.
Fig. 2
The results of simulation as a function of time of the accumulated doses given by radioactive nuclei 226Ra to 214Po (indicated by 226Ra), 210Pb to 206Pb (indicated by 210Pb), 228Ra and 228Ac (indicated by 228Ra), and 228Th to 208Pb (indicated by 228Th). The values are calculated for HPD1621R03, taken at a sea-floor hydrothermal area at depth of 1495 m of Hatoma Knoll, Okinawa Trough, the ESR age of which was estimated to be 33 years while the bulk concentrations of 226Ra and 228Ra are estimated to be 17.0 Bq/g and 59.4 Bq/g, respectively.
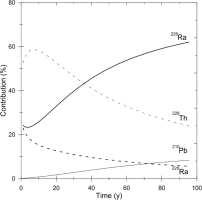
From the values shown in (Fig. 2), the contributions of the accumulated doses are calculated as a function of time and shown in % (Fig. 3) from 226Ra to 214Po, 210Pb to 206Pb, 228Ra and 228Ac, and 228Th to 208Pb, indicated by 26Ra, 210Pb, 228Ra, and 228Th, respectively. The contribution from 228Th to 208Pb reaches 60% at its maximum and remains still 30% at 100 years when the nuclei have already decayed out.
Fig. 3
The contributions to the accumulated dose as a function of time of the nuclei, 226Ra to 214Po (indicated by 226Ra), 210Pb to 206Pb (indicated by 210Pb), 228Ra and 228Ac (indicated by 228Ra), and 228Th to 208Pb (indicated by 228Th) in %, calculated from the values in Fig. 1.
For a longer time scale, the contribution to the accumulated dose from 228Ra series (228Ra to 208Pb) is calculated as shown in Fig. 4, which is in logarithmic time scale. As shown in the figure, even after 300 years, the contribution from 228Ra series is sti11 10%. It is obvious that the ESR ages calculated without contribution of 228Ra series to the accumulated dose will be overestimates. For the present test sample, a simulation indicates that the age will be estimated to be 90 years at age of 50 years when the contribution is not taken into account, while it will still be 360 years, 20% overestimates, at age of 300 year, where the overestimate is comparable as the typical variation, 20%, of the ESR ages from a same portion (Fujiwara et al., 2015) including the measurement error. The overestimate becomes 5% at age of 500 years.
Fig. 4
The contribution to the accumulated dose from 228Ra series as a function of time in logarithmic scale.
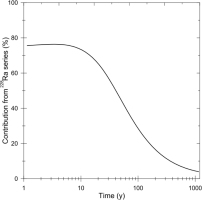
As the half-life of 228Ra is 5.75 year, although depending on the sample, the nucleus can be detected for young samples. For such young samples, the contribution of the dose from 228Ra series can be taken into account and the ESR age can be appropriately estimated. For the current test sample, the 228Ra concentration will become 7×10−5 Bq/g comparable as the detection limit after 50 years. After this age, the contribution cannot be estimated, because 228Ra series nuclei have decayed out, but still necessary to be considered according to the above discussion. In summary, the ages of the samples for which the ESR ages have been estimated to be less than 300 years without considering the doses from 228Ra series, would have probably been overestimated and this would most typically happen for the samples older than 50 years because 228Ra cannot be detected.Currently, any date over 50 years should be considered a maximum value when the 228Ra content is not obtained.
How to correct this contribution would be an important but difficult issue. The initial 228Ra concentration needs to be estimated. For this particular sample examined in the present study, the initial 228Ra/226Ra activity ratio is estimated to be 3.49 from the present 226Ra and 228Ra concentrations and the ESR age, which is equivalent to virtually equilibrated Th/U ratio (in ppm) of 10.7. This ratio would be a little high but in the reasonable range as material in the crust. One way would be to investigate and accumulate data on the initial 228Ra/226Ra activity ratio in young or preferably zero age barite samples or in the hydrothermal fluid.
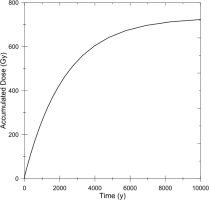
The upper age limit of ESR dating has not been discussed except for the thermal stability, which was estimated to be at least several thousand years (Sato et al., 2011), while the oldest age was reported to be 16000 years (Fujiwara et al., 2015). According to the present simulation of the accumulated dose for further longer time scale, it is found that the dose will be close to saturation after about 5000 years as shown in Fig. 5, in which the sum of the accumulated doses from all 226Ra and 228Ra series nuclei is calculated as a function of time. This is clearly less than ten times the half-life of 226Ra, which is 1600 years. As 226Ra decays, the dose rate gets lower and the accumulated dose increases only slightly. Considering the errors in estimating DE, the age would not correctly be estimated for those more than 5000 or 6000 years old. High precision methods may become available in the future. Until then, when the ESR age appears to be older than 6000 years, it should be considered just to be older than 6000 years.
. Conclusions
As results of simulation of the doses from the radioactive nuclei of 226Ra and 228Ra series, the contribution from 228Ra series can be significant for samples younger than 300 years. As for the present test sample, the contribution from 228Th to 208Pb was calculated to reach 60% at its maximum and to remain still 10% at 300 years. Currently, the ages for these samples without considering doses from 228Ra series would be overestimates. It is necessary to estimate the initial 228Ra concentration in barite in order to obtain the correct ages, and therefore, this is the further issue to be investigated especially for the samples older than 50 years old, in which 228Ra is not detected. Systematic investigations on the 228Ra/226Ra activity ratio in young or zero age samples would help estimate this value. The age limit of ESR dating of barite would be about 5000 to 6000 years due to decay of 226Ra, rather than the thermal stability of the signal.